|
BrainDrugs is a 7½-year (2019-2026) thematic research alliance within precision medicine in epilepsy and depression. The project is funded by the Lundbeck Foundation (40 mio DKK grant) and anchored at Neurobiology Research Unit with Prof. Gitte Moos Knudsen as Director. |
|
Background
There is currently an enormous unmet need for the development of effective precision medicine approaches for brain disorders. More precise treatment strategies are needed to replace the present “one-size-fits-all” and subsequent “trial-and-error” approach currently applied in our patients. An important step to achieve this goal is to uncover endophenotypes and biomarkers that can critically help to stratify patient cohorts (see figure below).
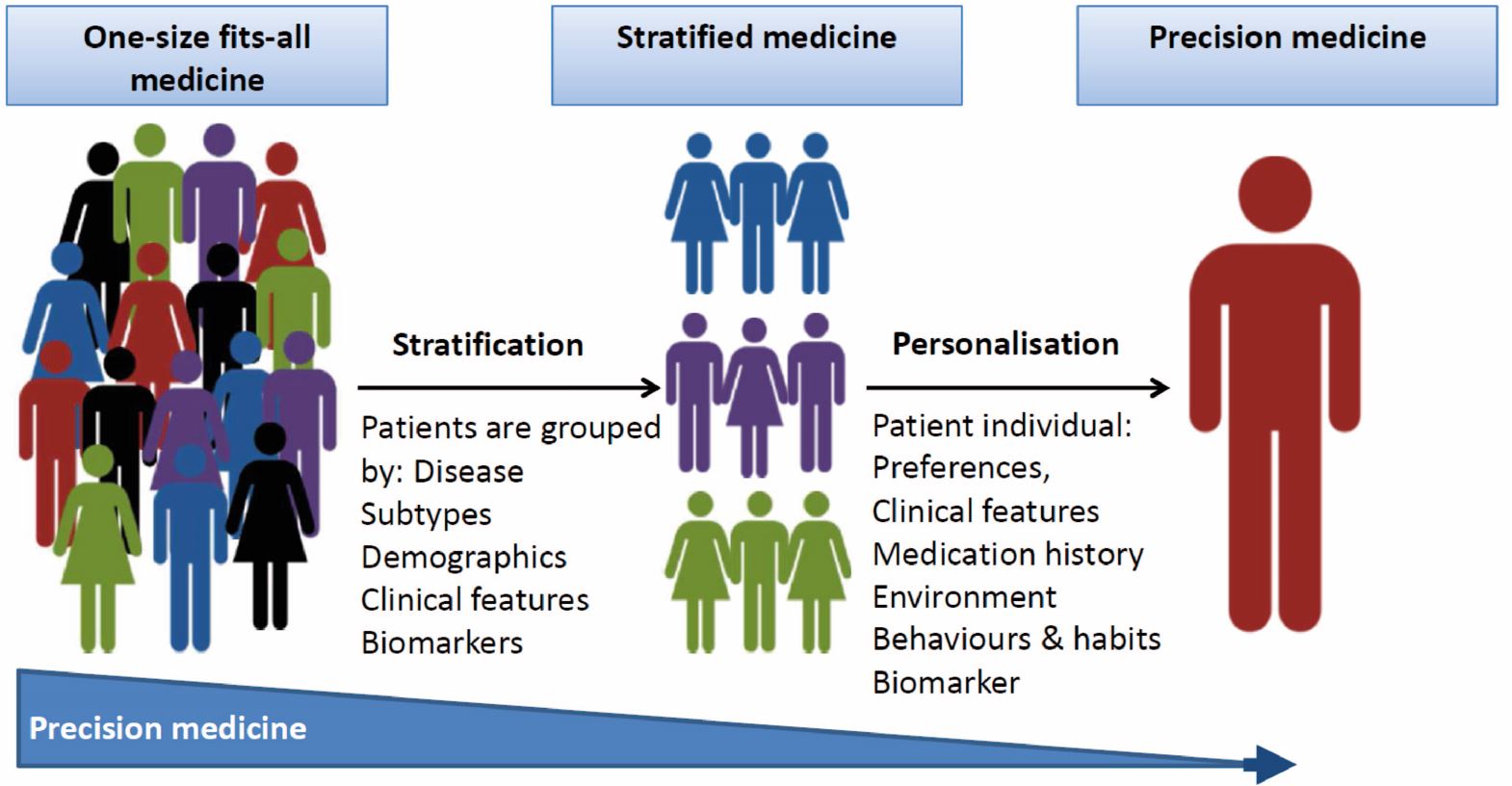
To enable stratification of patients based on their underlying pathogenetic and pathophysiological brain disturbances will provide “enriched” patient populations that are more likely to benefit from specific interventions. Stratification will also be enormously beneficial for interpretation of clinical trials that aim to test new compounds and is essential to allow for an optimised treatment of patients with brain disorders. Another important challenge is to determine the target engagement of pharmacological interventions, and to predict occupancy based on, e.g., plasma drug concentrations.
With BrainDrugs, we will focus on two frequent and devastating brain disorders: epilepsy and major depression (MDD). We have chosen to study epilepsy and MDD because they are frequent and devastating brain disorders with significant comorbidity and because both conditions are treated with pharmacological interventions that often fail to succeed, including failure due to intolerable adverse effects. Epilepsy often co-occurs with mood disorders and there is reason to believe that the comorbidity is underrecognized in both patient groups. Comorbidity is also associated with less favorable outcome of drug treatment. That is, there is a huge unmet medical need to better diagnose and treat these two serious brain disorders.
Initially, we aim at understanding the mechanisms which explain the variation in drug response using a diverse set of methodologies, including standard epidemiological biostatistical approaches with national registry data and machine learning based on big data from routine care, e.g., clinical (symptom) characterization, neuroimaging, or genetics. The discovery phase will likely identify features of drug resistance, which will be used to characterize groups of patients and identify clusters of factors associated with comparable types of drug responses. From this, we will formulate a series of drug response indices that when validated, can be used to personalise drug interventions and to establish a recommendation for treatment of the individual patient.
Methods and Materials
We will make use of Danish registries to identify associations between intake of brain targeting drugs and clinical outcomes in order to uncover which patient features define a successful antidepressive or antiepileptic drug treatment. To investigate if we can identify patient subgroups, we will conduct advanced text-mining analyses which extract specific clinical features from electronic patient records. Through existing deep phenotyping or genetic databases with biobanks, those features will be related to, e.g., genetic and neuroimaging data to define biologically valid patient subgroups suffering from depression and epilepsy. To increase power to detect biomarkers and treatment response, we will establish new cohorts of patients with epilepsy and depression that we follow longitudinally.
Expected outcome and perspectives
It is our ambition to set the stage for a precision medicine approach in pharmacological treatment of epilepsy and depression, for the benefit of future patients. To succeed in our mission, we have devised a strategy for implementation of research outcomes in the clinic. It is our hope that in the long run, BrainDrugs can serve as a model to be implemented internationally, and for other brain disorders.
